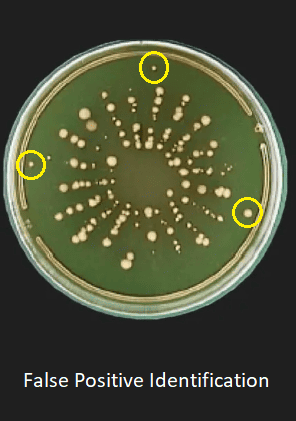
Reduce fake positives in clean microbial air sampling
In controlled environments such as clean space, keeping sterility is very important. One of the most important processes in this context is Microbial air sampling– A method used to detect contaminants in the air that can endanger the process of making sterile. However, a significant challenge in sampling of microbial air is a risk Positive positive in a clean room. This fake positive can cause unnecessary investigations, stop time, and potential loss of products, making it an expensive problem for industries such as medicines, biotechnology, and health care.
In this blog, we will explore how modern innovations, such as Biocapt® Single Use Microba Impactor, can increase the efficiency of microbial air sampling while reducing the risk of fake positive in clean rooms.
The role of microbial air sampling in a clean room
Microbial air sampling plays an important role in ensuring the clean space environment remains not contaminated. This process involves capturing microorganisms in the air in agar plates or in special devices. After sampling, microorganisms collected are incubated, and every microbial growth is analyzed. The aim is to ensure that clean air is free from contaminants that can endanger the sterility of the product or the manufacturing process.
In traditional methods, microbial air sampling often involves stainless steel, which requires manually handling plates. During this process, there is a risk of polluting plates, which lead to fake positive – agencies where the sample shows contamination that is actually not in the environment. This fake positive can create unnecessary concerns and additional workloads because they trigger investigations into non -existent contamination.
Positive false in cleanrooms: expensive problems
Fake positive in a clean room has a problem because they cause unneeded interventions and expensive delays. In an environment where even the smallest contamination can have a dramatic impact – such as pharmaceutical manufacturing or critical health care environment – positive false can cause stop production, expensive investigations, and significant financial losses.
In many cases, this false positive is caused by operator errors or contamination during manual handling of sampling equipment, such as when the operator touches or manipulates the plate so that during the microbial air sampling process. For industries that rely on sterile conditions, minimizing human intervention and potential contamination is the key to ensuring accuracy in the results of microbial sampling.
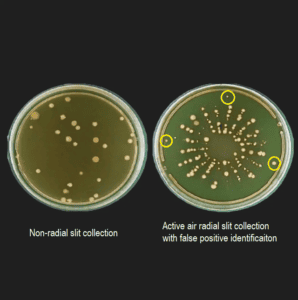 How biocapt use microbial impact microbes reduce positive wrong
How biocapt use microbial impact microbes reduce positive wrong
BIOCAPT The use of microbes The impact of microbes discusses many challenges that lead to fake positives in sampling of microbial air. This innovative device is specifically designed to reduce human intervention, which in turn reduces the possibility of contamination during sampling.
The main features of the Biocapt use of microbial microbes include:
- Plates so that sterile that has been published before: Unlike the impact of traditional stainless steel, which requires operators to manually place and handle agar plates, the single use of this biocaptation is equipped with a sterile plate that has been published before. This eliminates the need for manual intervention, drastically reduces the risk of contamination of human errors.
- Sterile design, irradiated gamma: All sterile and triple-Bag devices, undergo gamma irradiation before use. This ensures that microbial pools remain free from contaminants during the sampling process.
- Disposable comfort: After use, the beam biocapt can be removed, eliminating the need for cleaning that takes time or sterilization of equipment that can be reused. It also eliminates the risk of contamination of incorrect equipment.
- Reducing operator treatment: By integrating the plates to be in the threat itself, the use of the BIOCAPT disposable reduces the need for operator interventions. This minimizes the contact point where contamination can occur, helping to reduce the occurrence of false positive.
Increased efficiency and compliance with sampling
In addition to reducing fake positive, the impact of Biocapt versatile microbes offers high sampling efficiency, ensuring that microbial air sampling remains effective in detecting potential contaminants. The design of the looping gap makes it possible for the right sampling of air microorganisms, ensuring that the efficiency of physical and biological collection is maximized.
Biocapt Single Use Microba Impactor comply with industrial standards, including ISO 14698-1, which outlines the principles and methods for controlling biocontamination in a clean room environment. By meeting this strict standard, Biocapt Impactor ensures that sampling data can be relied upon and can be followed up, increasing efforts to control clean contamination.
Conclusion
For industries where sterility cannot be negotiated, such as drugs and aseptic manufacturing, the risk caused by fake positives in sampling of microbial air is significant. These mistakes not only cause unnecessary investigations and downtime production but also threaten the integrity of sensitive manufacturing processes.
Innovative solutions such as Biocapt Single Use Microba Impactor offer a way forward by reducing the potential contamination induced by operators, increasing sampling efficiency, and ensuring compliance with industrial standards. By adopting such technology, the operator of hate rooms can improve the process of taking microbial air sampling and reducing the occurrence of expensive false positive, ultimately ensuring a safer environment, more efficient, and free of contamination.
TLDR; Takeaways Key:
- Microbial air sampling is very important to detect contaminants in the air in a sterile environment such as a clean chamber.
- Fake positive in a clean room can be caused by operator handling errors during sampling, which leads to unnecessary costs and delays.
- Biocapt® Single Again Microbial Impactor reduces false positive by eliminating manual handling of agar plates and using a sterile design and published before.
- By increasing the sampling efficiency and reducing the risk of contamination, the disposable Biocapt® helps ensure compliance with cleanroom standards, including ISO 14698-1.
- By focusing on modern progress in sampling of microbial air, clean space operators can significantly reduce false positive risk, ensuring a more reliable and efficient contamination control process.
Want to learn more?
Download the full paper here
News
Berita
News Flash
Blog
Technology
Sports
Sport
Football
Tips
Finance
Berita Terkini
Berita Terbaru
Berita Kekinian
News
Berita Terkini
Olahraga
Pasang Internet Myrepublic
Jasa Import China
Jasa Import Door to Door

